Metabolomic engineering and synthetic biology in biotechnology
Bacteria that have been metabolically modified using synthetic biology are already used to produce many chemicals. Phototrophic micro-organisms, cyanobacteria, microalgae and anoxygenic phototrophic bacteria, which use the freely available energy of sunlight and inorganic carbon for growth and thus contribute to the reduction of the atmospheric CO2 concentration, represent a very attractive basis for biotechnological applications. However, their wider use is still limited by the low production efficiency resulting from the lack of suitable synthetic biology tools.
In our project, we are developing a working prototype of a photosynthetic biofactory, Syn2Cell. It is a cyanobacteria-based system characterized by high productivity achieved by reprogramming metabolic pathways using synthetic biology and adaptive laboratory evolution. By further stabilizing the photosynthetic processes and genetic makeup, we will achieve higher robustness of the system, which is essential for practical biotechnological applications.
The project also includes the development of a technological procedure for culturing the cell system in pilot volumes of tens of litres.
We are further focused on the development of synthetic algal biology, including the identification of enzymes important for the targeted expression of high-value metabolites with application potential.
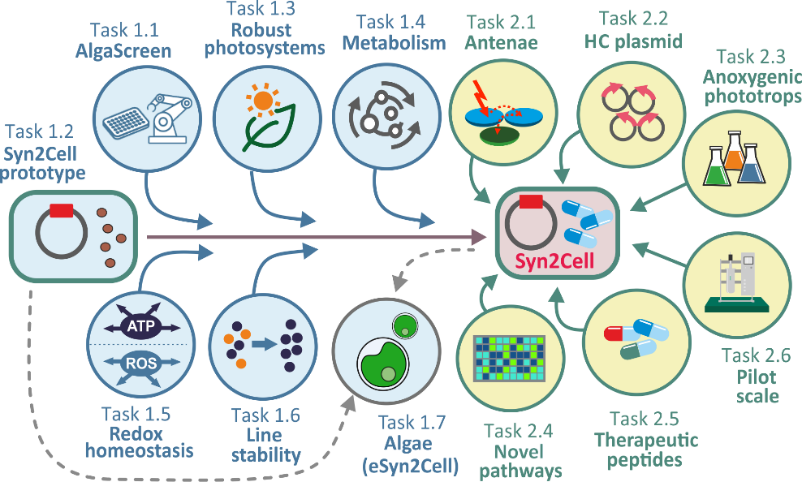
The main objective of this workpackage is to construct a conceptually novel production system called Syn2Cell, based on the model cyanobacterium Synechocystis. Using synthetic biology and adaptive laboratory evolution, the original microbial cell will be reprogrammed so that the growth phase is separated from the production phase and the system is sufficiently robust. The production phase will be initiated by blocking growth and triggering a built-in metabolic program that allows the cell to survive in nitrogen-free conditions. However, this program will be genetically modified so that it can be activated in the presence of nitrogen. The activation of this program will lead to the degradation of light-harvesting antennae (phycobilisomes) and the accumulation of storage substances. The carbon and nitrogen released by the degradation of the phycobilisomes will ensure very high production. Since all the necessary nutrients will be abundantly available, activation of this programme will not inhibit photosynthesis.
At the same time, our aim is to deepen the understanding of the regulation of algal metabolism, which is much more complex than in cyanobacteria (epigenetics, existence of organelles, etc.). We aim to establish a theoretical basis for the construction of a Syn2Cell-like system in eukaryotes.
The lack of suitable synthetic biology tools currently limits the wider use of microscopic phototrophs for biotechnological purposes. WP 2 aims to develop an expression system for cyanobacteria based on an inducible plasmid with a high copy number.
WP 2 builds on WP 1 to construct synthetic chlorophyll-binding proteins to replace the light-harvesting function of phycobilisomes in the Syn2Cell production system. This system will be tested for large-scale production of cyanobacterial secondary metabolites for pharmacology. Specifically, these will be substances with inhibitory effects on intercellular bacterial communication and potential anti-cancer pharmaceuticals. In addition to these priority substances, the metabolic pathways of carotenoids, flavonoids and other substances with biotechnological potential will be characterized in phototrophic microorganisms.